The results of the partitioning experiments
Our experiments exhibited significantly different behavior in the series of experiments. In the H2O-CO2 experiments, which employed the leaching method to determine the Mo concentration of the fluid, the DMofluid/melt (CMofluid/CMomelt, in which CMofluid and CMomelt represent the concentration of Mo in the equilibrated fluids and quenched glasses, respectively) was 0.2 ± 0.02 (2σ) at XCO2 = 0.1, 0.34 ± 0.04 and 0.4 ± 0.04 (2σ) at XCO2 = 0.15 and 0.25 ± 0.02 (2σ) at XCO2 = 0.2 (Supplementary Table 5) (where XCO2 represents the mole ratio of CO2/(CO2 + H2O) in the experiments). Thus, values of the partition coefficient for Mo do not correlate with XCO2 in NaCl-free systems involving H2O-CO2 fluids (see below).
In the H2O-NaCl experiments, all the fluid inclusions had the same vapor-liquid ratio at room temperature (Fig. 1a). Based on the equation of state for the H2O-NaCl system at the experimental conditions (850 °C, 200 MPa), the fluid lies in the single-phase region. Values of DMofluid/melt, calculated using the concentration of Mo in the leaching solution, ranged from 0.51 ± 0.28 (2σ) to 21.3 ± 10.3 (2σ), whereas DMofluid/melt values, calculated using the concentration of Mo in fluid inclusions, ranged from 0.66 ± 0.33 (2σ) to 25.7 ± 14.0 (2σ). The two sets of DMofluid/melt values, however, are indistinguishable within the analytical uncertainty of ~ 20% (Supplementary Fig. 2b). In general, the DMofluid/melt value increases with increasing salinity (Fig. 2b). At low salinity (≤20 wt.%), the DMofluid/melt values (calculated using the fluid inclusion data) vary from 0.66 ± 0.33 to 3.18 ± 1.41 (2σ), whereas at higher salinity, and especially at a salinity above that of halite saturation, the partition coefficient of molybdenum increases sharply, from 6.08 ± 2.48 (2σ) at a NaCl concentration of 21.2 wt.% to 25.7 ± 14.0 (2σ) at a NaCl concentration of 44.3 wt.% (Supplementary Table 5).
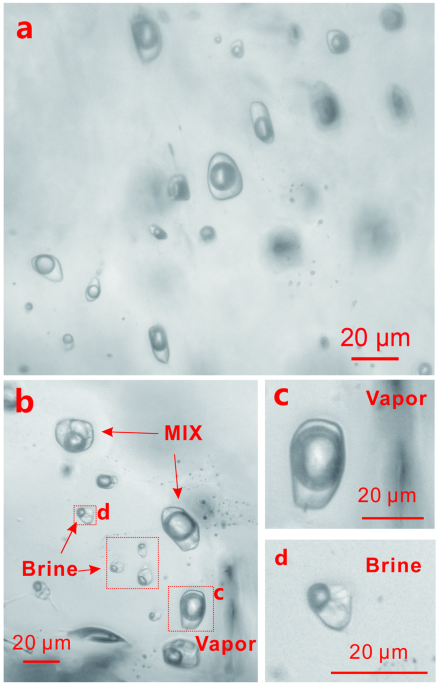
Fig. 1: Photomicrographs of fluid inclusions trapped in quartz during experiments at 850 °C and 200 MPa.
a Supercritical H2O-NaCl fluid inclusions from experiment Q-006. b Coexisting low-density vapor inclusions, high-density brine inclusions, and heterogeneously trapped vapor and brine inclusions in the H2O-NaCl-CO2 system from experiment Q-016. c, d are enlargements of one of the vapor and brine inclusions in (b), respectively.
Fig. 2: The DMo vs. XCO2 and salinity of fluid.
a DMofluid/melt vs. XCO2 for the H2O-CO2 experiments. The DMofluid/melt values were calculated from the leaching solution analyzed by ICP-MS and quenched melt analyzed by LA-ICP-MS. b DMofluid/melt vs. salinity in the H2O-NaCl and H2O-NaCl-CO2 experiments. The solid squares represent the DMofluid/melt values calculated from the concentrations of Mo in fluid inclusions and the quenched melt of the H2O-NaCl experiments. The blank circles represent the DMofluid/melt values calculated from the leaching solution and quenched melt of the H2O-NaCl experiments, and the solid diamonds represent the partition coefficients for Mo between brine and melt for the H2O-NaCl-CO2 experiments. Because of the large uncertainty in the estimates of the salinity of the vapor fluid inclusions in the H2O-NaCl-CO2 experiments, only maximum and minimum values of DMovapor/melt are shown. The solid cycle represents the average value of the above value. c An enlargement of the rectangular box in (b) showing the values of DMofluid/melt for the high salinity experiments in the H2O-NaCl-CO2 system. The corresponding XCO2 is also indicated.
In the H2O-NaCl-CO2 experiments, brine and vapor were trapped as separate (immiscible) phases as shown by the presence of vapor and brine inclusions, as well as heterogeneously (variable proportions of vapor and brine), in quartz from the experiments for this system (Fig. 1b, c, d). This indicates that the melt equilibrated with separate brine and vapor phases in this system at the experimental conditions (850 °C, 200 MPa). As a result of this phase separation, the salinity of the brine increased from that of the homogeneous system (~ 7 wt.%) to 56 wt.% NaCl at a XCO2 of 0.1 and 62 wt.% NaCl at a XCO2 of 0.3. The DMobrine/melt value increased from 81 ± 41.1 to 179 ± 69.7 (2σ) as the salinity increased from 56 wt.% NaCl (XCO2 = 0.1) to 62 wt.% NaCl (XCO2 = 0.3) (Fig. 2c and Supplementary Table 5).
The influence of salinity on D
Mo
The results of this study clearly show that the partition coefficient of Mo between hydrothermal fluids and felsic magmas (DMofluid/melt) increases with the salinity of the fluid and that this increase is exponential with salinity (Fig. 2b, c). Thus, hypersaline fluids are very efficient in leaching Mo from coexisting felsic melts. Several studies have reported trends of DMofluid/melt values with salinity similar to that reported here15,16,17,18. In all cases the DMofluid/melt value for the high salinity fluids was reported to be ≥100 times higher than that for low salinity fluids. These data are illustrated in Fig. 3 and are consistent with experimental and theoretical findings that Mo is transported dominantly by the species NaHMoO40 (ref. 21) or, at very high temperatures, by the species MoO2(OH)Cl (ref. 24), for a wide range of salinity. We, therefore, conclude that any process that leads to the formation of a hypersaline brine in equilibrium with a fertile felsic magma will lead to an ore fluid that is enriched in Mo and capable of the deposition of this metal in concentrations sufficient to produce economic porphyry Mo deposits.
Fig. 3: Compilation of DMofluid/melt between fluids and melts from experiments with different temperatures and pressure, and 2 sigmas standard deviation of the measurements was illustrated.
The experimental data was collected from Candela and Holland13, Webster15, Tattitch, and Blundy16, Fang and Audetat17, Zhan et al.18, and this study. The values increase exponentially with salinity. The values reported by Tattitch and Blundy16, and Fang and Audetat17 are consistently higher than those of the current study. A possible reason for this is the presence of sulfur, which is interpreted by the authors of these papers to increase the solubility of Mo in the fluids relative to that of Mo in sulfur-free experiments.
The role of CO2 on D
Mo
The results of our study show that CO2 is not complex with Mo (Fig. 2a) and thereby increases the ability of aqueous carbonic fluids to extract Mo from magma. On the contrary, our experiments with CO2-H2O fluids show that an increase in the CO2 content of the system does not affect the Mo content of the fluid or the fluid-melt partition coefficient for Mo, which were both very low (Supplementary Table 5). The same conclusion was reached by Li et al.23, However, as we show below, CO2 plays a very important, albeit indirect, role in the leaching of Mo from felsic melts and the formation of Mo-rich ore fluids.
In experiments with H2O-NaCl-CO2 fluids, the fluid separated into immiscible brine and vapor at a temperature of 850 °C and 200 MPa, provided that the XCO2 was greater than 0.1. At lower XCO2 and in the system H2O-NaCl system at the same temperature and pressure there is a single supercritical fluid (Fig. 4a), consistent with the observation of Li et al.25, that the addition of CO2 expands the two-phase region of the H2O-NaCl fluid system and promotes fluid immiscibility.
Fig. 4: The phase diagrams of H2O-NaCl and H2O-NaCl-CO2, extracted efficiency of Mo from exsolved fluid and schematic diagrams of the formation of Dabie-type porphyry molybdenum deposits.
a A pressure-salinity diagram showing phase relationships in the systems H2O-NaCl, and H2O-NaCl-CO2 calculated from Driesner and Heinrich43 and Duan et al.44, respectively. b The extraction efficiency ratio of molybdenum, defined as the mass of Mo in the exsolved fluid phase divided by the mass of Mo in the initial magma, versus the ratio of exsolved fluid to magma. The DMofluid/melt values obtained from our experiments were used to calculate the extraction efficiency. The blue line represents the extracted efficiency of Mo from initial magma when XCO2 = 0.1, and 0.3, respectively. The Black dashed line represents the extracted efficiency calculated for the peraluminous system. The red line represents the extracted efficiency in H2O-NaCl supercritical fluid. Details of the calculation are provided in the supplemental materials. c The schematic diagram illustrated the post-collisional extensional environment45 of Dabie-type or Collision-type porphyry molybdenum deposits. d The diagram shows the fluid evolution mole for Dabie-type porphyry molybdenum deposits, featuring immiscible vapor and brine CO2 bearing ore-forming fluids that directly exsolve from felsic magma.
The maximum value of DMobrine/melt determined in this study for the H2O-NaCl-CO2 system is ~ 100 times higher than that determined for an H2O-NaCl fluid having the same bulk NaCl content. The reason for this is the very strong partitioning of NaCl into the liquid and the resultingly low NaCl content of the coexisting vapor. Consequently, the liquid in the H2O-NaCl-CO2 system will have a much higher NaCl content than the supercritical fluid in an H2O-NaCl system with the same NaCl content as that of the combined fluids in the H2O-NaCl-CO2 system. As the DMofluid/melt value of the brine increased sharply with NaCl content at the higher end of the range of NaCl contents considered, it follows that the DMofluid/melt of the brine in the H2O-NaCl-CO2 system will reach a value many times higher than that for the corresponding H2O-NaCl system.
An important feature of the behavior of CO2 in magma is that it promotes the saturation of the magma with fluid26,27,28. Moreover, it also promotes the exsolution of the fluid as separate CO2-rich vapor and NaCl-rich liquid because, as mentioned above, the immiscibility region in the system H2O-NaCl-CO2 increases with increasing XCO2 (ref. 27). Thus, at the temperature-pressure conditions of the emplacement of magmas forming Dabie-type porphyry Mo deposits (850 °C and 200 MPa), the magma will exsolve an ore-forming hydrothermal fluid as separate (immiscible) vapor and brine phases, even if the bulk fluid contains as little as 10 mole % CO2.
In summary, the principal findings of our study are: 1) that DMofluid/melt values increase with increasing NaCl content of the fluid, particularly at the upper end of the range of NaCl contents considered; and 2) that, for the temperature-pressure conditions of our experiments (similar to those for the emplacement of the magmas forming Dabie-type porphyry Mo deposits) and XCO2 > 0.1, the melt coexists with vapor and a hypersaline brine with a higher NaCl content than that of the supercritical fluid in the H2O-NaCl system having the same bulk NaCl content. The main implication of these findings is that liquid-vapor phase separation induced by the presence of CO2 during the exsolution of H2O-NaCl fluids from magmas may be the key to the efficient extraction of Mo from a fertile magma and the formation of an economic Dabie-type porphyry Mo deposit.
Implications for Dabie-type porphyry molybdenum ore formation
As mentioned in the introduction to this paper, Dabie-type porphyry molybdenum deposits, which postdated the Qinling-Dabie continent-continent collision, are the main Chinese source of Mo, accounting for over half of the resource of this metal in China and one-third of the resource globally. In contrast to the hydrothermal fluids that formed Climax- and Endako-type porphyry molybdenum deposits, the hydrothermal fluids responsible for the formation of Dabie-type porphyry Mo deposits are of higher temperature, higher salinity, and CO2-rich4. Indeed, CO2-bearing vapor inclusions and coexisting halite-bearing fluid inclusions are ubiquitous in Dabie-type porphyry Mo deposits, e.g., the Shapinggou deposit, the largest Mo deposit in Asia and the Tangjiaping deposit, another large Mo deposit8,10.
Like the fluid inclusions trapped during the formation of Dabie-type Mo deposits, those trapped during our experiments with the H2O-NaCl-CO2 system comprise assemblages of coexisting CO2-rich and halite-bearing liquid-vapor inclusions. Accordingly, we propose that the hydrothermal fluids forming Dabie-type porphyry Mo deposits exsolved directly from the magma as separate CO2-rich vapor and hypersaline brine phases. This contrasts with the cases of the Climax- and Endako-type deposits, for which the common occurrence of intermediate density fluid inclusions below the deposits and separate brine and vapor inclusions at the level of the deposits provide evidence that the corresponding magmas exsolved a single supercritical fluid that subsequently underwent phase separation29,30,31.
To evaluate the efficiency with which a brine in the system H2O-NaCl-CO2 can extract Mo from a magma, we calculated the extraction efficiency using the DMofluid/melt values determined from our experiments (details of the calculation are provided in the Supplementary S5). The results of this calculation show that when 10 % of a two-phase fluid with a brine/vapor (CO2) ratio of 0.1 and a bulk salinity of ~ 7 wt.% is exsolved directly from the magma, it will extract 62.3% and 85.3% of the Mo from the magma at a XCO2 of 0.1 and 0.3, respectively. In contrast, a supercritical fluid with a NaCl content of 6.3 wt.%, will only extract 17.0 % of the Mo from the magma. Thus, the efficiency of extraction of Mo by a brine in the H2O-NaCl-CO2 system containing a separate vapor is 3.7 ~ 5 times higher than that of supercritical fluid in the H2O-NaCl system with the same bulk salinity as the H2O-NaCl-CO2 system. In addition, if the extraction efficiency is calculated using the ASI of the peraluminous magmas associated with porphyry molybdenum deposits (the DMo for peraluminous melts is approximately half that for peralkaline melt17,19), the extraction of Mo is still 2 – 3 times higher than that with supercritical H2O-NaCl fluids (Fig. 4b).
Based on the results of our experiments and the extraction efficiency of Mo from magmas, we envisage the following scenario for the genesis of Dabie-type porphyry molybdenum deposits. Ore formation begins with the production of a fertile magma from the partial melting of crustal material due to upwelling of the asthenosphere4,32 (Fig. 4c). This magma is enriched in CO2 because of the elevated carbonate content of the crust, in NaCl because of an evaporite component or sediments9,32 and in Mo because of the incompatible behavior of Mo during the generation of the crust from the mantle. On emplacement at a depth corresponding to ~ 200 MPa, this magma undergoes CO2-induced fluid exsolution and, because of the high CO2 content, exsolves a CO2-rich vapor and a separate hypersaline liquid, which sequesters the Mo. Finally, owing to the overpressures created by the fluid exsolution, fractures are created into which the liquid is dispersed to form a quartz-molybdenite stockwork (Fig. 4d). We propose that the scenario envisaged here satisfactorily explains the formation of Dabie-type Mo deposits and note that the exsolution of a two-phase fluid is supported by the boiling or effervescent fluid inclusion assemblages that characterize most Dabie-type porphyry Mo deposits8,10,11.
To conclude, the results of our experiments show that the H2O-NaCl-CO2 fluids in equilibrium with felsic magmas at the conditions of emplacement of Dabie-type deposits occur as separate CO2-rich vapors and hypersaline brines, that the unusually high salinity of the brines results in extremely high fluid/melt partition coefficients for Mo, and that this enables extremely efficient extraction of Mo from the magma. Accordingly, we propose that CO2 plays an important, if not controlling role in the formation of Dabie-type porphyry Mo deposits by inducing fluid-exsolution at greater depths than would be possible for Climax- or Endako-type deposits and by ensuring that two fluids are exsolved. The result is a hypersaline brine that has the high NaCl content needed to extract most of the Mo from the magma and facilitate the formation of a Collisional- or Dabie-type porphyry Mo deposit.