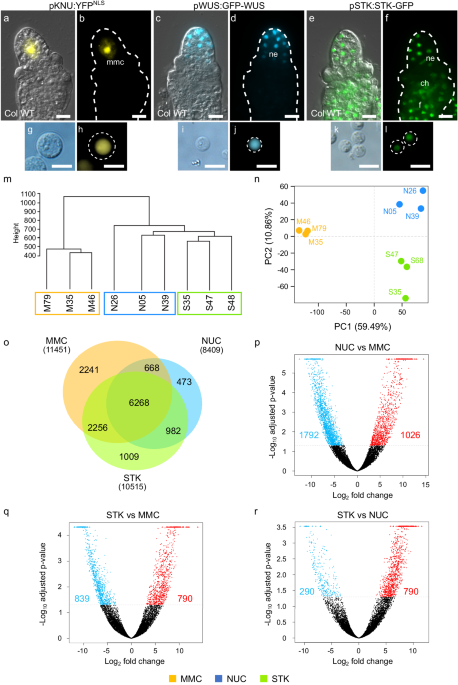
Transcriptional analysis of germline and somatic cells reveals distinct gene expression profiles
To explore the molecular pathways that contribute to female germline development we generated and characterised different ovule cell type-specific transcriptomes. We used marker lines that define the MMC (pKNU:YFPNLS18; Fig. 1a, b), nucellus (pWUS:GFP-WUS18; Fig. 1c, d) and most somatic cells of the ovule (pSTK:STK-GFP19; Fig. 1e, f) coupled with sorting of fluorescent protoplasts. Protoplasts were isolated from ovules at stage 2-II when callose starts to accumulate around the expanding MMC. Enzymatic separation of the ovule cells produced pKNU:YFPNLS protoplasts that varied in diameter from 8 to 14 µm (hereafter termed the “MMC” sample; Fig. 1g, h). By comparison, pWUS:GFP-WUS (“NUC” sample) and pSTK:STK-GFP protoplasts (“STK” sample) varied in size from 5 to 8 µm in diameter (Fig. 1i–l). Only protoplasts showing strong nuclear signals were collected for RNA extraction (Fig. 1h, j, l). Using these stringent parameters, yields were low but consistent. For example, we typically obtained around 4 intact, fluorescent pKNU:YFPNLS protoplasts per 40 ovules (i.e. the average number of ovules per flower).
Fig. 1: Cell-type specific transcriptional profiling of young Arabidopsis ovules.
a, b pKNU:YFPNLS ovule, the yellow fluorescent protein is detected solely in the megaspore mother cell (mmc) nucleus. c, d pWUS:GFP-WUS ovule, the WUS-GFP fusion protein is detected in the nucellus epidermis (ne). e, f pSTK:STK-GFP reporter protein is observed in the somatic cells of the ovule, in the nucellus epidermis (ne), chalaza (ch) and funiculus (f). Protoplasts produced from pKNU:YFPNLS (g, h), pWUS:WUS-GFP (i, j), and pSTK:STK-GFP (k, l) ovules showing fluorescent protein expression restricted to the nucleus. a, c, e Images result from merging bright-field DIC with YFP/GFP fluorescence. b, d, f Fluorescence channel only. Scale bars: 10 μm. RNAseq quality assessment: m Hierarchical clustering dendrogram and n Principal Component Analysis (PCA) scatter plot using read count matrixes calculated by DESeq2 method. o Venn diagram showing the number of genes expressed in each cell type (in parenthesis) and the overlap between transcriptomes. p–r Volcano plots depicting the differentially expressed genes for each comparison (blue: downregulated genes = FDR < 0.05, log2 (fold change) < −2; red: upregulated genes = FDR < 0.05, log2 (fold change) > 2). y axis – adjusted p-value is the FDR value calculated as described in the methods section. M35, M46, M79 = pKNU:YFPNLS samples (MMC) biological replicates; N05, N26, N39 = pWUS:GFP-WUS (NUC) biological replicates; S35, S47, S68 = pSTK:STK-GFP (STK) biological replicates.
RNA extraction from 20 to 40 protoplasts per biological replicate and subsequent RNA sequencing generated on average 7.5 million reads per sample, and over 70% of these reads were uniquely mapped to the Arabidopsis TAIR10 genome (Supplementary Table 1). To investigate whether the transcriptomic profiles were distinguishable by cell type, unsupervised hierarchical clustering and principal component analysis (PCA) were conducted. The clustering analysis showed that the MMC biological replicates form a cluster independent of NUC and STK replicates (Fig. 1m). Regarding PCA, two components explain most of the variability and underlie the separation of the replicates into three groups according to their cell type (Fig. 1n). While PC1 separates the MMC samples from the samples of somatic origin, PC2 allows separation of the NUC and STK samples. In summary, both analyses show that the transcriptomic profiles are distinguishable based on sample origin and, importantly, a clear distinction is detected between the germline and somatic transcriptomes.
The number of expressed genes was similar across transcriptomes, ranging from 8400 to 11500 genes (Fig. 1o). Of these, 6268 genes were commonly expressed in the three samples. Differential gene expression analysis (Supplementary Dataset 1) revealed the highest number of differentially expressed genes (DEGs) between NUC and MMC, i.e., 2818 genes (Fig. 1p). The comparison between STK and MMC showed a total of 1629 DEGs (Fig. 1q), whereas the lowest number of DEGs were identified between STK and NUC, with a total of 1288 genes (Fig. 1r). Subsequent analysis of the DEGs revealed cell-type specific expression profiles consistent with previous published transcriptomic data, in situ hybridisation experiments, reporter lines and/or immunolocalisation experiments (Supplementary Fig. 1)3,4,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32. Highly restricted expression patterns were confirmed for genes expressed solely in the MMC (Supplementary Fig. 1a, f, g), the nucellus (Supplementary Fig. 1b), and within the somatic domain defined by STK protoplasts (Supplementary Fig. 1c, d). We could also demonstrate that transcripts of genes known to be expressed throughout the ovule were detected in all three transcriptomes (Supplementary Fig. 1e). A direct comparison of our data, generated from fluorescence-sorted protoplasts, to the recently published unsupervised scRNAseq6 of young Arabidopsis ovule protoplasts (Supplementary Table 2) showed overlaps in the MMC cluster. This provided confidence that our method can be used to extract key differences between specific ovule cell types, such as the MMC and adjoining nucellus.
Gene set enrichment analyses highlight differences in plasmodesmal signalling between ovule cell types
To define processes occurring in each cell type, a Gene Set Enrichment Analysis was performed for each sample pair combination (see Fig. 2 for a selection of relevant GO terms and Supplementary Dataset 2 for full lists of GO terms).
Fig. 2: Enriched gene ontology terms in MMC, nucellar (NUC) and somatic (STK) transcriptomes.
Selection of the biological processes and cell component GO terms comparing NUC and MMC (a), STK and MMC (b), and STK and NUC samples (c). Numbers at the end of the bars indicate the number of genes for each GO term. d, e pKNU:PDLP1a-GFP is expressed in a punctate pattern in the megaspore mother cell (mmc) walls. In d, purple colour shows autofluorescence. f, g pAGO5:PDLP1a-GFP is observed in the nucellar epidermis (ne) and inner integument (ii) cells. d, e Widefield fluorescence microscopy. f, g Laser Scanning Confocal Microscopy, max-projection of three GFP slices (green). Experiments were repeated at least 4 times for d, e, and three times for f, g, with similar results and representative micrographs are shown. Scale bars = 20 μm.
In comparison to the other samples, the MMC transcriptome clearly showed enrichment of terms related to meiosis, negative regulation of mitosis, cell cycle checkpoint, and vesicle-mediated transport. Relative to NUC, the MMC also showed enriched terms such as gene expression (transcription, translation and maturation) and RNA splicing (Fig. 2a; Supplementary Dataset 2). Conversely, relative to STK there were enriched terms in the MMC related to protein transport, secretion, and cell growth (Fig. 2b).
In the NUC transcriptome we found enriched GO terms related to cell communication, cell wall, signal transduction, plasmodesmata and symplast, compared to the MMC and STK samples (Fig. 2a, c). Additional terms related to cell differentiation and the endomembrane system, such as Golgi apparatus and endoplasmic reticulum, appeared when comparing NUC with STK, but not with the MMC (Fig. 2c; Supplementary Data 2). The NUC and MMC comparison uncovered terms associated with cell wall organisation and biogenesis (Fig. 2a; Supplementary Data 2), while the STK samples were always enriched for terms related to gene expression (Fig. 2b, c; Supplementary Data 2). Additionally, in the STK and MMC comparison, we detected terms related to cell wall, cell communication, response to hormone (namely auxin and brassinosteroid), plant ovule development, plasmodesma, and symplast (Fig. 2b). This shows some overlap with the NUC transcriptome, which is not unexpected considering the STK transcriptome also contains nucellar cells.
These results confirm that the overall molecular signatures are distinct and characteristic of each cell type. They also reveal that symplastic intercellular signalling pathway genes are enriched in the “nucellus” transcriptome relative to the adjoining germline cells. Indeed, from the 2818 genes differentially expressed between MMC and NUC, 267 gene transcripts are predicted to encode proteins that localise to PD33, accounting for about 9% of all DEGs. Because little is known about the regulation of symplastic transport components in ovules, we explored this pathway in greater detail.
Genes related to PD composition and function are expressed in specific ovule cell types
First, we considered the location of PD in the ovule tissues of interest. PLASMODESMATA-LOCALIZED PROTEIN1a34 has been extensively used as a PD marker when fused to GFP (PDLP1a-GFP35,36). Importantly, PDLP1a is not normally expressed in the ovule (Supplementary Fig. 2), hence it forms a useful marker protein that is unlikely to interfere with endogenous PDLP1a activity. Expression of PDLP1a-GFP under the KNU promoter (pKNU:PDLP1a-GFP), which is exclusively detected in the MMC, showed a weak but punctate localisation pattern at the cell periphery, and was most abundant at the proximal pole (Fig. 2d, e). A pAGO5:PDLP1a-GFP construct directed GFP expression to the nucellar epidermis and inner integument primordia, and consistent with previous marker studies, was absent from the MMC (Fig. 2f, g). In the epidermis, PDLP1a-GFP protein was predominantly detected in anticlinal walls and assumed a punctate pattern consistent with PD localisation. PDLP1a-GFP was not obvious in the innermost wall of the epidermal cells that adjoin hypodermal cells, including the MMC at the distal tip. These data support previous TEM studies that suggest the MMC and NUC both contain PD15.
Next, we investigated candidate genes influencing PD composition and connectivity. Glucan synthases (GSLs) and β−1,3-glucanases (BGs) control the synthesis and hydrolysis of callose at PD37 and are key determinants of the PD size exclusion limit (SEL) that influences intercellular movement. Additionally, the receptors encoded by PDLPs and PLASMODESMATA CALLOSE BINDING PROTEINS (PDCBs) localise in membranes and are thought to promote callose deposition14. Most of these putative regulators of PD permeability showed restricted expression in the germline or somatic transcriptomes (Supplementary Figs. 2 and 3). For example, expression of GSL2 was confined to the MMC (Supplementary Fig. 3a–c), while GSL4 was detected in ovule cells other than the MMC (Supplementary Fig. 3a, d, e). PDLP and PDCB transcripts were detected only in the NUC and STK transcriptomes, consistent with the enrichment of symplastic pathways in those cell types (Supplementary Fig. 2).
Notably, the proportion of BGs and GSLs expressed in the MMC varied when compared to surrounding cells (Supplementary Fig. 3). Six out of nine expressed GSLs were abundant in the MMC. Of these, GSL1, GSL5 and GSL10 are predicted, and GSL8 is confirmed, to localise to PD33. GSL3 and GSL6 showed elevated expression in NUC and STK and are also predicted to locate to PD33. By contrast, only three out of 13 detected BG were transcriptionally enriched in the MMC. Most of the BG genes were expressed specifically in the NUC/STK sample (Supplementary Fig. 3), with AT1G66250, AT3G13560, AT2G01630 previously confirmed, and AT4G29360, AT3G55430, AT3G07320 predicted, to be located in PD33,38.
Taken together, the cell-specific transcriptional profiles suggest that genes involved in callose biosynthesis, callose hydrolysis, and PD permeability are abundant and differentially expressed in the MMC compared to surrounding cells.
Intercellular movement assays confirm the existence of a germline-specific symplastic domain in the ovule
The expression of multiple GSL genes in the MMC coincides with a stage when the MMC becomes symplastically isolated from long-distance pSUC2:GFP moving into the ovule from the phloem16. However, whether the inhibition of movement depends on callose accumulation, or has any implications for germline development, remains unclear. To address this further, we aimed to develop a system for tracking molecule movement in and out of the MMC. Initially we tested the previously described long-distance pSUC2:GFP system for its ability to unload GFP from the phloem into the ovule. Unfortunately, we were unable to replicate pSUC2:GFP movement in our conditions. Instead, we developed cell-type specific markers to examine the details of local symplastic connectivity between the germline and surrounding cells. A gene encoding a mobile mStrawberry protein (mStrfree) was expressed under the control of the pAGO5 and pKNU promoters. In wild-type (WT) plants, the cell-autonomous endoplasmic reticulum (ER)-localised pAGO5:YFPER protein is unable to move between cells and accumulates in the nucellar epidermis, inner integument and chalaza, but is excluded from the MMC and funiculus (Fig. 3a, b18). In plants expressing mStrfree under the same pAGO5 promoter (pAGO5:mStrfree), a similar but broader fluorescent signal was observed. Apart from being detected in the nucellar epidermis and chalaza, the mStrfree signal was observed near the proximal funiculus but not in the inner integument or the MMC (Fig. 3c, d). This confirms that the mStrfree protein can move in a distal to proximal direction in the ovule, but cannot enter the MMC at the stage when callose deposits are present. Conversely, expression of nuclear localised (NLS) cell-autonomous YFPNLS protein from the pKNU promoter was detected in the MMC, but not the nucellus (Fig. 3g, h18). In WT plants, mobile pKNU:mStrfree accumulated to high levels in the MMC (Fig. 3i, j), and, similar to the cell-autonomous pKNU:YFPNLS protein, did not spread into surrounding nucellar cells, confirming symplastic isolation of the MMC.
Fig. 3: Localisation of cell autonomous and mobile fluorophores in wild-type and pKNU:GLUC ovules.
a, b In wild-type ovules, cell-autonomous pAGO5:YFPER accumulates in the nucellar epidermis and inner integuments. c, d pAGO5:mStrfree shows a broader pattern in the chalaza and nucellus but is excluded from the MMC and is absent in the inner integuments. e, f A similar pattern is observed in pKNU:GLUC ovules. g, h In wild-type ovules, cell-autonomous pKNU:YFPNLS accumulates in the nucleus of the MMC. i, j. pKNU:mStrfree spreads throughout the MMC but does not spread into the nucellar epidermis of wild-type ovules. k, l A similar pattern is observed in pKNU:GLUC ovules in the majority of cases. m-r In approximately 18% of pKNU:GLUC ovules, pKNU:mStrfree signal is apparent outside of the MMC in adjoining nucellus and chalaza cells (arrows in n, p and r). Experiments were repeated on 5 independent occasions with similar results. Representative micrographs are shown. Scale bars = 20 µm. ch chalaza, f funiculus, ii inner integument, mmc megaspore mother cell, ne nucellar epidermis, oi outer integument.
Next, we considered the possibility that movement across the MMC-NUC interface is influenced by a stringent PD size exclusion limit. Previous studies have used 8-hydroxypyrene-1,3,6-trisulfonic acid trisodium salt (HPTS) as a mobile tracer dye to assess symplastic connectivity16,39,40, and in contrast to the ~27 kDa mStrfree protein, HPTS is only around 0.5 kDa in size. Intercellular movement of HPTS into ovules was tested using dissected inflorescence stems from WT plants. HPTS moved rapidly into the stem and upwards into flowers. In WT ovules, HPTS moved into the ovule and accumulated in defined spots within the funiculus and chalaza during germline development but was not detected in the nucellus or germline cells (Fig. 4a–f).
Fig. 4: Localisation of HPTS tracer in wild-type, pKNU:GLUC and pWUS:GLUC ovules.
a–f In wild-type, HPTS accumulates in the funiculus and chalaza of pre-meiotic and post-meiotic ovules. g–l A similar localisation pattern is observed in pKNU:GLUC ovules. m–r In pWUS:GLUC ovules, HTPS accumulates strongly in the funiculus and chalaza, progressing further towards the nucellus. Unlike the other tested genotypes, signal is occasionally detected in the vicinity of the mmc/megaspores. The red dashed lines indicate the interface between different ovule domains. Experiments were repeated on 4 independent occasions with similar results. Representative micrographs are shown. Scale bars = 20 µm. ch chalaza, dm degenerating megaspores, fu funiculus, ii inner integument, mmc megaspore mother cell, mt megaspore tetrad, ne nucellar epidermis, nu nucellus. oi outer integument.
Overall, these mobility assays confirm a degree of symplastic connectivity between ovule cells, and symplastic barriers at the chalaza-funiculus and the germline-nucellus boundaries. The findings are consistent with earlier studies and provide new tools to investigate genes and pathways influencing symplastic connectivity in the ovule.
Identification of an atypical β−1,3-glucanase that alters callose deposition in vitro and in vivo
Callose is a classic marker for the MMC and megaspore tetrad and can be detected using decolourised aniline blue (DAB) and/or immunolabelling41. Consistent with the cell-specific gene expression data, WT ovules containing expanding MMCs (stage 2-II) showed callose accumulation in the MMC wall as spots and occasionally in the cell plate of adjoining nucellar cells (Fig. 5a). When the MMC was fully expanded (stage 2-III), DAB staining revealed larger aggregates at the cell periphery that eventually encompassed the entire cell wall (Fig. 5c, e). During meiosis, callose was most abundant in walls separating the megaspores, and became concentrated in the degenerating megaspores, at the base of the FM, prior to the initiation of gametogenesis (Supplementary Fig. 4a). No DAB staining was detected in the developing gametophyte where mitosis occurs in the absence of cytokinesis (Supplementary Fig. 4b, c).
Fig. 5: Callose deposition and phenotypes in ovules expressing GLUC.
a, c, e In wild-type, decolourised aniline blue (DAB) stains punctate callose deposits in the wall of the MMC as it expands from stages 2-II to 2-III. These deposits accumulate over time and become large aggregates that encompass much of the MMC wall. b, d, f In pKNU:GLUC ovules, punctate callose deposits are less obvious in the MMC wall and the overall pattern is more diffuse compared to wild-type. Despite this, large aggregates are detected at the cell periphery at stage 2-III prior to the initiation of meiosis. g Cleared wild-type ovule containing a wild-type mature embryo sac at anthesis. h Example of a wild-type-like embryo sac from a pKNU:GLUC line. i Aborted FG1 embryo sac (where the functional megaspore has aborted gametogenesis at FG1 with a central nucleus and vacuoles at both poles) in a pKNU:GLUC ovule. j Aborted FG1 embryo sac in a pWUS:GLUC ovule. Staging according to Schneitz et al.5: (a, b) stage 2-I, (c–f) stage 2-II to 2-III. Experiments were repeated on 6 independent occasions with similar results. Representative micrographs are shown. Scale bars = 20 µm. aFG1 aborted female gametophyte, ccn central cell nucleus, ecn egg cell nucleus, DAB decolourised aniline blue, FGm mature female gametophyte, fu funiculus, ii inner integument, mmc megaspore mother cell, ne nucellar epidermis, oi outer integument, sc seed coat, sy synergid.
To address whether callose deposition in the MMC is required to enforce its symplastic isolation, and whether this is needed for germline development, we used several approaches. First, we examined plants carrying mutations in the MMC-enriched GSL genes, but found that in our growing conditions, callose was still present in the MMC wall. We speculate that complete inhibition of callose biosynthesis in the MMC may require a higher-order cell-type-specific gsl mutant. Another strategy to modify callose levels involves ectopic-expression or mutation of BG gene, which has previously been reported to decrease or increase callose deposition, respectively, affecting cell-to-cell movement of molecules, as well as growth, development, and fertility42,43,44,45. We therefore considered whether cell-type specific expression of a BG might be sufficient to disturb callose accumulation and the germline-nucellus symplastic barrier. To avoid potential problems with endogenous gene silencing in Arabidopsis, we searched for suitable BG genes from other species. Previous reports described the GLUC gene (Supplementary Fig. 5a, b) as a putative BG from Hieracium piloselloides that is expressed during megasporogenesis46 and shares homology with the Arabidopsis anther-specific At4g14080 (A6) and At3g23770 (A6-like1; A6-L1) genes (Supplementary Fig. 5a). The A6/GLUC sequences reside within the α-clade of the GH17 BG family47 and contain an N’-terminal signal peptide but lack a glycosylphosphatidylinositol (GPI)-anchor. In contrast to the Arabidopsis A6-like proteins, the predicted GLUC amino acid sequences from Hieracium and other Asteraceae species also lack a C’-terminal X8 carbohydrate-binding domain (Supplementary Fig. 4b, 5a). Thus, GLUC is similar but not identical to BGs associated with reproductive callose dissolution in Arabidopsis.
To test the enzymatic activity of GLUC, the protein was expressed in E. coli. A filtrate of control and GLUC expressing cultures was incubated with a range of substrates including cellohexaose, laminarihexaose, xyloglucan, 1,3;1,4-β-glucan, lichenan, curdlan, laminarin and yeast β-glucan. Incubation with GLUC led to the release of glucose from 1,3-β-glucan substrates that contain 1,3-linkages only, but was unable to cleave 1,3-linkages in substrates such as barley 1,3;1,4-β-glucan (Supplementary Fig. 5c). This suggests that GLUC encodes a functional BG.
Next, we investigated the sub-cellular location of GLUC, which was predicted to be extracellular based on DeepLoc 2.048. The coding sequence was fused to GFP and expressed in Allium cepa (onion) and Nicotiana benthamiana (tobacco) epidermal cells using the constitutive CaMV 35 S promoter. In onion cells, GLUC-GFP accumulated in strands of ER located around the nucleus and throughout the cell (Supplementary Fig. 6e). GLUC-GFP was also located in punctate spots at the cell periphery adjoining the plasma membrane, indicating that the enzyme is likely to be secreted and may accumulate in PD (Supplementary Fig. 6f, g). This location closely resembled that of other proteins previously confirmed to accumulate and function in PD49. GLUC-GFP localisation was also examined in tobacco leaf pavement cells, where PD are easily identified by DAB staining of callose50. Approximately 50% of the punctate spots labelled with DAB also showed GLUC-GFP signal (Supplementary Fig. 6h–j), suggesting that GLUC co-locates at least partially with the PD. Quantification of punctate callose deposits indicated that cells expressing 35S:GLUC-GFP or 35S:GFP-GLUC showed a reduced frequency of DAB staining relative to plants expressing 35S:GFP (Supplementary Fig. 6k). In agreement with the in vitro assays of the recombinant GLUC enzyme, these results demonstrate that GLUC is able to hydrolyse PD callose in vivo.
To address the effects of GLUC expression on callose deposition in the ovule, two constructs were generated; a germline-specific pKNU:GLUC gene to target the germline-nucellus symplastic block, and a nucellus-specific pWUS:GLUC gene, with a broader zone of action including the whole nucellar epidermis adjoining the chalaza. Ovules from transgenic plants were analysed by DAB staining in comparison to WT.
DAB staining patterns in pWUS:GLUC ovules appeared similar to WT with regards to the timing and amount of callose in the MMC wall, although the labelling occasionally appeared diffuse at stage 2-II (compare Supplementary Figs. 7a and 5c). Callose was also detected in ovules from pKNU:GLUC plants, but fluorescence intensity measurements confirmed a significant reduction in callose accumulation in the MMC compared to WT (Supplementary Fig. 8). Detailed analysis revealed that the appearance of punctate callose deposits in the MMC wall at ovule stage 2-II was consistently delayed (Fig. 5b). This was also confirmed by immunolabelling with an anti-callose antibody (BS400-2; Supplementary Fig 9). Thin sections of ovules from WT plants highlighted punctate callose deposits in the wall between the MMC and adjoining nucellar epidermal cells at stages 2-I and 2-II (Supplementary Fig. 9a–c, e). By contrast, in ~40% (n = 63 ovules) of the pKNU:GLUC ovule sections that contained an MMC, callose deposits were not detected or infrequently detected in the MMC wall (Supplementary Fig. 9d, f). Although the callose labelling was initially reduced or delayed in pKNU:GLUC ovules (Fig. 5d), accumulation of other cell wall components, as detected by the LM20 (methylesterified pectin)51 and JIM13 (arabinogalactan proteins)52 antibodies, appeared unchanged. Moreover, callose was eventually detected in the MMC wall of all ovules. Indeed, apart from the early differences, the subsequent stages of megasporogenesis in both pKNU:GLUC and pWUS:GLUC showed similar patterns of callose deposition and DAB staining intensity to WT (Supplementary Fig. 4d–f; Supplementary Fig. 7c, d; Supplementary Fig. 8). Taken together, these results suggest that callose deposition in the MMC wall is transiently modified in pKNU:GLUC ovules during MMC expansion. Defects in callose deposition were less obvious in pWUS:GLUC lines, and were not investigated further here.
Cell type-specific expression of GLUC leads to defects in female gametogenesis and local changes in symplastic connectivity
The pKNU:GLUC and pWUS:GLUC lines were indistinguishable from WT in terms of plant height and growth habit. However, analysis of ovule development indicated that both constructs compromised female gametogenesis (Fig. 5g–j; Supplementary Table 3). In WT, approximately 98% (n = 3335) of the ovules at anthesis contained a mature female gametophyte, including an egg cell and central cell nucleus (Fig. 5g). At the same stage of development, ~39% of the ovules in pKNU:GLUC lines (n = 3288) had aborted at the first stage of gametogenesis (FG1; Fig. 5i; Supplementary Table 3), while the remainder appeared normal (Fig. 5h). Heterozygous pKNU:GLUC plants were emasculated and crossed with WT pollen to assess whether FG1 abortion results from somatic activity (i.e. expression in the MMC), or transgene activity that segregates during meiosis (i.e. gametophytic activity; Supplementary Table 4). Analysis of progeny confirmed that 54% (n = 59) of the F1 plants carried the pKNU:GLUC transgene. This suggests that germline abortion is not due to expression of pKNU:GLUC in the FM or female gametophyte, but is consistent with pKNU:GLUC affecting development of the unreduced diploid MMC.
The frequency of germline abortion in pWUS:GLUC lines was consistently lower than pKNU:GLUC lines, but similar defects were detected. Approximately 25% of the pWUS:GLUC ovules (n = 1620) showed abortion at FG1 (Fig. 5j; Supplementary Table 3). Hence, the quantitative (pKNU:GLUC) and qualitative (pWUS:GLUC) differences in callose deposition detected by DAB staining and immunolabelling are accompanied by defects in female germline development.
To assess if the defects correlate with changes in tracer molecule mobility, and thus destabilisation of the MMC-NUC symplastic barrier, we utilised the fluorescent reporters described above. pAGO5:mStrfree localisation was similar in pKNU:GLUC and WT ovules, whereby the marker was unable to enter the MMC (Fig. 3e–f). Conversely, pKNU:mStrfree did not spread outwards from the MMC in WT or the majority of pKNU:GLUC ovules (Fig. 3k, l). However, in approximately 18% (n = 343) of pKNU:GLUC ovules, mStrfree signal was clearly detected outside of the normal pKNU domain, either in the chalaza and/or nucellar cells flanking the MMC (Fig. 3m–r). To quantify this, we measured the relative fluorescence intensity in the MMC and adjoining nucellar cells (Supplementary Fig. 10). The ratio of NUC:MMC fluorescence was significantly increased in the presence of pKNU:GLUC from (0.39 ± 0.12) to (0.52 ± 0.16). Altogether, these observations suggest that pKNU:GLUC expression leads to protein movement out of the MMC, and thus partially compromises the germline-nucellus symplastic block.
The small tracer molecule HPTS did not move beyond the chalaza in pKNU:GLUC lines, similar to WT (Fig. 4g–l). However, in pWUS:GLUC plants, at least 15% (n = 753) of the ovules reproducibly showed increased HPTS staining intensity and mobility. Intense signal was detected in the chalaza compared to WT and pKNU:GLUC, and extended upwards towards the base of the nucellus (Fig. 4m–r). While no signal was detected in the MMC or meiotic tetrad of WT or pKNU:GLUC plants, HPTS signal was weakly detected in pWUS:GLUC lines in the vicinity of the MMC during expansion, and became stronger during megaspore selection (Fig. 4m–r). Collectively, these results suggest that changes in callose deposition and germline viability induced by GLUC expression are accompanied by local changes in fluorescent tracer mobility.
pKNU:GLUC expression leads to changes in MMC-identity and epigenetic regulatory pathways
To assess whether cell identity is altered by pKNU:GLUC expression, we examined a range of cell-type specific markers in developing ovules. Analysis of the pWUS:GFPNLS and pPIN1:PIN1-GFP markers in pKNU:GLUC ovules revealed a similar expression domain to WT, suggesting there was no obvious change in epidermal or pro-vascular cell identity (Supplementary Fig. 11a–d). Similarly, the number of ovules expressing the pKNU:YFPNLS marker in the MMC was unchanged in pKNU:GLUC plants compared to WT, suggesting that features of MMC identity are maintained (Supplementary Fig. 11e, f). We also used immunolabelling to examine H3K27me1 histone marks, since previous studies indicated that this mark is typically present in most ovule cells but not the MMC or FM3. Immunolabelling of thin sections from WT ovules at stage 2-II confirmed previous findings from wholemount studies, whereby labelling was detected in the nuclei of cells in the chalaza and nucellus, but was weak or undetected in the nucleus of the MMC (n = 3/48 ovules; 6.25%; Supplementary Fig. 12a, b). In pKNU:GLUC ovules, labelling was similar to WT in the chalaza and nucellus. However, labelling was notably different in the MMC whereby 45% (n = 37/80) of ovules showed clear immunolabelling in the MMC nucleus (Supplementary Fig. 12c). This was confirmed using samples from different laboratories and growing conditions. Taken together, these findings suggest that although cell identity appears to be generally normal in pKNU:GLUC ovules, a significant proportion of MMCs exhibit an abnormal “mixed” identity.
Finally, in order to assess these defects at a transcriptomic level, we conducted transcriptome (RNAseq) analysis on WT and pKNU:GLUC pistils (Supplementary Table 5) at the stage when callose deposition, mStrfree movement and histone labelling are altered. Expression of approximately 18000 genes was detected in both genotypes, and hierarchical clustering and PCA analysis clearly distinguished pKNU:GLUC samples from WT (Supplementary Fig. 13a–c). The overall Log2(fold-change) observed for most genes was generally low, almost never reaching the value of 1 or −1, consistent with localised changes occurring in the ovule. Therefore, genes showing an FDR value ≤ 0.05 were considered DEGs (Supplementary Dataset 3). Using this criterion 349 genes were upregulated in pKNU:GLUC relative to WT and 133 were downregulated (Supplementary Fig. 13d). These expression trends were confirmed for multiple genes using qPCR (Supplementary Fig. 13e).
GO term enrichment analysis revealed a range of biological process terms overrepresented in the pKNU:GLUC DEGs, including terms for meiosis, cell cycle transition regulation, DNA and RNA metabolism (Supplementary Dataset 4; Supplementary Fig. 14, 15). These terms were also found to be overrepresented in the MMC transcriptome (relative to NUC and STK; Fig. 2). Moreover, underrepresented biological process terms in the pKNU:GLUC DEGs appeared to be most similar to the terms enriched in the nucellus (Fig. 2), including cell wall biogenesis (e.g., xyloglucan, pectin, and oligosaccharide metabolic process), response to hormone, cell communication and the secretory pathway (Supplementary Fig. 14, 15). Further analysis of the cellular component terms revealed that genes encoding proteins involved in chromatin remodelling were overrepresented in the DEGs, and included components of the SWI/SNF (switch defective/sucrose nonfermentable) and SWR1 (SWI2/SNF2‐related 1) complexes53,54 (Supplementary Fig. 15). For example, BRAHMA (BRM), the hub of the SNF/SWI complex55, and another complex partner, SWI3A, were upregulated in pKNU:GLUC pistils (Supplementary Fig. 13f). From the SWR1 complex we also found upregulation of SWC4 and ACTIN RELATED PROTEIN 9 (ARP9). Other upregulated genes included LIN52A, a member of the DREAM complex that controls cell cycle transitions56, AURORA3 (AUR3), a kinase important for chromosome segregation57, RAS ASSOCIATED WITH DIABETES PROTEIN 51C (RAD51C), essential for homologous recombination during meiosis58,59, and ASYNAPTIC3 (ASY3), required for meiotic cell cycle crossovers60 (Supplementary Fig. 13f). The changes in transcript abundance are consistent with deregulation of pathways involved in MMC development, such as epigenetic reprogramming via chromatin remodelling3 and the transition to meiosis. These differences in expression appear to have remarkably little impact on overall ovule identity (Supplementary Fig. 11a–d), but coincide with transient deficiencies in symplastic MMC isolation, MMC identity, and dramatic downstream consequences for germline development.